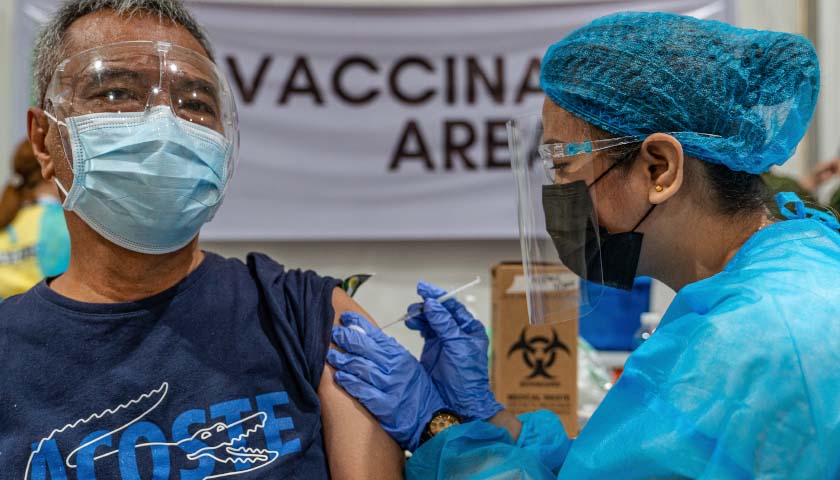
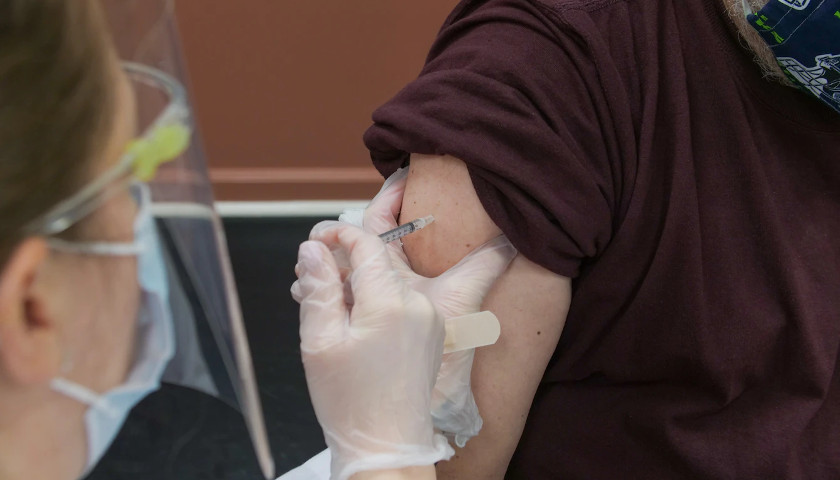
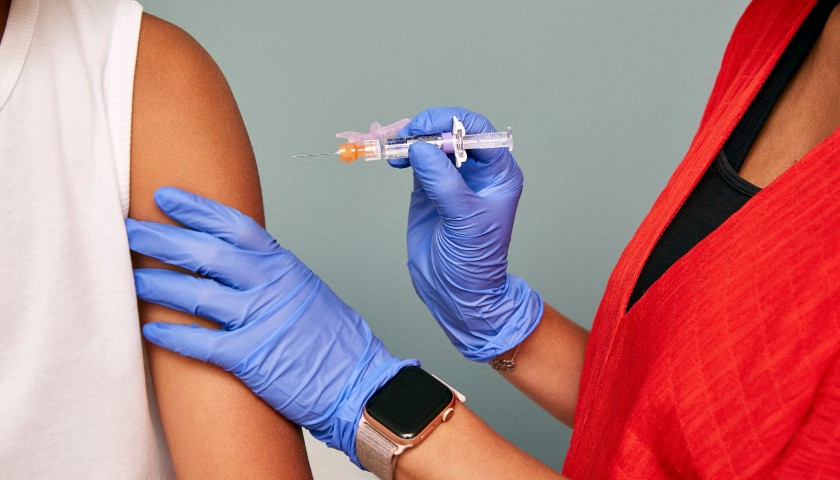
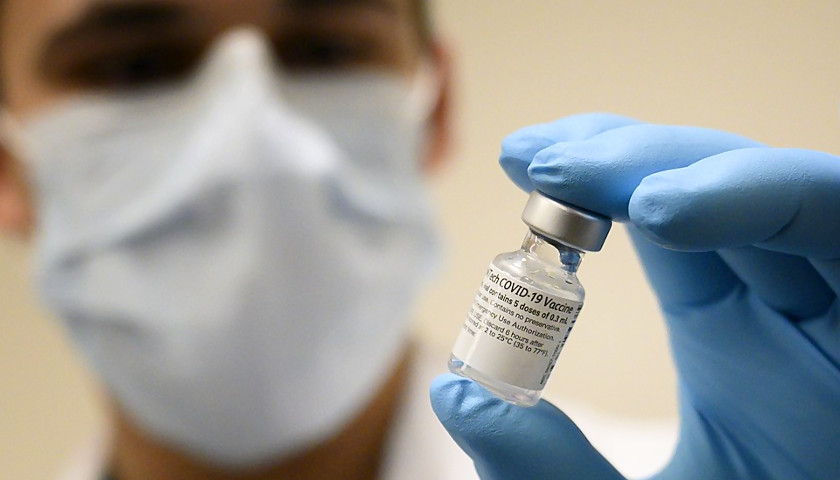
The U.S. Food and Drug Administration gave full approval Monday to the Pfizer COVID-19 vaccine, a major step that will likely have significant implications for vaccination mandates nationwide. The Moderna and Johnson & Johnson vaccines have not yet received full FDA authorization.
The Pfizer vaccine previously received FDA authorization, which allowed its emergency use but did not give the full approval. Pfizer is the first company to receive full approval in the U.S.
Read More