President-elect Donald Trump on Thursday nominated 2024 Independent presidential candidate Robert F. Kennedy Jr. to lead the Department of Health and Human Services.
Read MoreTag: FDA
Commentary: Vaccine Ad Blitz Sidestepped Transparency Rules
“A bun in the toaster oven,” a woman exclaims off-camera, handing an ultrasound image to family members who erupt into tearful emotion over the news. “Oh my God!”
The touching baby announcement video then gets down to business as text appears on the screen amidst the ongoing celebration, suggesting the best way to stay alive for this joyous birth is by becoming vaccinated against COVID-19. “Why will you get vaccinated? … Because some people you just want to meet in person.”
Read MoreFDA Approves of Leaky Mpox Vaccine That May Cause Heart Inflammation in ‘About 1 in Every 175 Persons’
Late last month, the U.S. Food and Drug Administration (FDA) approved a monkeypox vaccine that is known to “shed from the vaccination site” and cause heart inflammation in about 1 in every 175 persons.
ACAM2000, made by Emergent BioSolutions, was developed to prevent monkeypox disease in individuals determined to be at high risk for mpox infection. But according to the FDA’s own medication guide for the product, the risks of the vaccine appear to outweigh the benefits.
Read MorePro-Vaccine Doctors Skeptical of New COVID-19 Boosters: ‘I’d Really Like to See the Data’
The Centers for Disease Control and Prevention is pushing new COVID-19 boosters, claiming that people who don’t stay “up to date” with shots – regardless of how many they’ve already taken – “are more likely to get very sick” while those who take them annually are “much less likely to get very ill, be hospitalized, or even die” from COVID.
The Democratic nominee for president is so committed to staying up to date on jabs that Vice President Kamala Harris made COVID boosting a requirement to work on her campaign, “unless otherwise prohibited by applicable law.” They can also ask the human resources department for a “reasonable accommodation … prior to reporting to an office location.”
Read MoreFDA Knew ‘Gender Affirming’ Puberty Blockers Increase ‘Suicidality’ in 2017, Promotes Them Today
Five months before the Food and Drug Administration issued a health warning on puberty blockers widely used off-label to treat minors with gender confusion, undermining a Department of Health and Human Services office that claimed “early gender affirming care is crucial to overall health and well-being,” an FDA leader acknowledged other health concerns.
Pediatric patients exposed to “gonadotropin-releasing hormone agonists,” most with central precocious puberty (CPP) and “a handful … transgender kids using the drugs off-label,” had an “increased risk of depression and suicidality, as well as increased seizure risk,” Division of General Endocrinology clinical team leader Shannon Sullivan told colleagues.
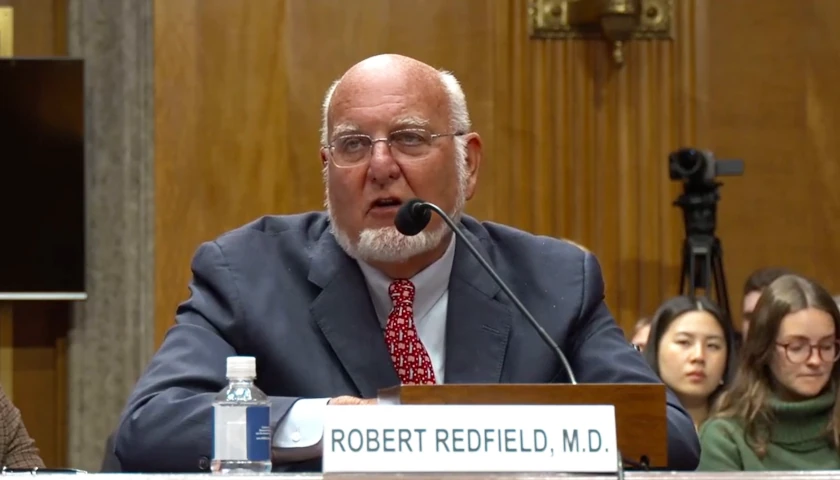
Read MoreFormer CDC Director Says FDA Underreported Adverse Side Effects of COVID Injections to Prevent Vaccine Hesitancy
Dr. Robert Redfield, the former director of Centers for Disease Control and Prevention (CDC) said Thursday that the U.S. Food and Drug Administration (FDA) pushed a false “safe and effective” COVID vaccine narrative by underreporting adverse events. The mRNA shots “never should have been mandated,” Redfield told the Senate Committee on Homeland Security and Governmental Affairs Committee on Thursday.
The Democrat-controlled Senate oversight hearing entitled “Risky Research: Oversight of U.S. Taxpayer Funded High-Risk Virus Research,” included witnesses Dr. Gerald Parker, Dr. Carrie Wolinetz, Dr. Kevin Esvelt, and Redfield.
Read MoreFDA Blesses Departing COVID Vaccine Reviewers to Influence Agency ‘Behind the Scenes’ at Moderna
David Morens, a senior adviser to then-National Institute of Allergy and Infectious Diseases Director Dr. Anthony Fauci, once told an ally “our FOIA lady” showed him “how to make emails disappear after I am FOIA’d but before the search starts.” He’s now on administrative leave.
Fauci’s former chief of staff, Greg Folkers, appeared to intentionally misspell proper nouns and names likely to be sought in Freedom of Information Act requests related to COVID-19 origins.
Read MoreFDA Vaccine Regulator Shunned COVID Booster, Warns the System Lets ‘Hierarchy Overrule Science’
A 30-year veteran of the Food and Drug Administration said at a congressional hearing this week he resigned in part because top brass sidelined his office to rush the full approval of Pfizer’s COVID-19 vaccine in August 2021, apparently to legally enable a vaccine mandate, then a booster under emergency use authorization over the objections of the agency’s outside advisers.
But former Office of Vaccines Research and Review Deputy Director Philip Krause perhaps saved his biggest embarrassment to the FDA for the end of Wednesday’s hearing on alleged Biden administration political interference in COVID vaccine review: He declined the booster.
Read MoreKansas AG Kris Kobach Sues Pfizer for Misleading Kansans About COVID Shots
Kansas Attorney General Kris Kobach announced Monday that he is suing Pfizer for misleading Kansans about its COVID mRNA shots.
Kobach is accusing Pfizer of deceiving the public about the significant health risks associated with the mRNA products, and of misrepresenting the efficacy of the jabs.
Read MoreFDA Agrees to Remove Anti-Ivermectin Posts Off the Internet in Lawsuit Settlement
The Food and Drug Administration (FDA) has reportedly settled a lawsuit brought by three doctors who accused the health regulator of interfering with their ability to practice medicine and prescribe Ivermectin to treat COVID.
Dr. Mary Talley Bowden, Dr. Paul E. Marik and Dr. Robert L. Apter sued the FDA in June of 2022, asking the court to: “Hold unlawful and set aside any FDA actions directing or opining on whether ivermectin should be used for certain off-label purposes, including treatment of COVID-19.”
Read MoreFDA Threatens Endangered Species with Shoddy Abortion-Drug Reviews: Lance Armstrong Investigator
Federal public health officials created strange bedfellows among animal-welfare advocates, scientists and vaccine skeptics for allegedly cutting corners in viral and COVID-19 vaccine research and oversight, possibly engineering a pathogen, then a cure that’s worse for some.
The Food and Drug Administration may be creating another odd couple in a case at the Supreme Court: environmental and pro-life activists.
Read MoreJust the News Sues Biden Administration to Force Disclosure of COVID-19 Vaccine Safety Data
Just the News on Thursday sued the Biden administration in federal court seeking to force the disclosure of COVID-19 safety data that is being kept outside the government’s normal adverse events reporting system
In the lawsuit filed in partnership with the America First Legal public interest law firm, Just the News asked the U.S. District Court in Washington, D.C., to order the Department of Health and Human Services to comply with two Freedom of Information Act requests to the Food and Drug Administration and the Centers for Disease Control and Prevention seeking COVID-19 reactions data kept in a back-end, nonpublic system to the nation’s Vaccine Adverse Event Reporting System (VAERS).
Read MoreFeds Conceal Details About Anti-Ivermectin Campaign in Response to Doctors’ Reinstated Lawsuit
The Food and Drug Administration wants to continue its selective promotion of off-label drug use: good for COVID-19 vaccines, bad for alternatives to those vaccines. It just doesn’t want the public to see its full reasoning for the latter.
The FDA and the Department of Health and Human Services filed a renewed motion to dismiss a lawsuit by doctors claiming the agencies have a practice of demonizing ivermectin by conflating its human and animal doses and using “command” language, such as “stop it,” to discourage using the anti-parasite drug against COVID.
Read MoreSupreme Court to Weigh Major Case on Abortion Pill Approval
The Supreme Court announced Wednesday that it is taking on a case regarding the Food and Drug Administration’s approval of the chemical abortion pill mifepristone.
Alliance for Hippocratic Medicine, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians and the Christian Medical & Dental Associations filed a lawsuit against the FDA in November 2022, claiming that the FDA had ignored safety protocols to approve the abortion pill mifepristone. The Supreme Court said this week that it would hear the case, one of the first major abortion cases taken up by the court since overturning Roe v. Wade in June 2022, according to an order list.
Read MoreFDA Downplays COVID Vax Overdosing as Hydroxychloroquine Shows More Promise in European Research
The FDA repeatedly told the public that an antiviral with a sterling safety record, ivermectin, should not be used to treat COVID-19 because it was also prescribed, at higher dosages, to livestock.
The agency didn’t appear to show the same concern about correctly dosing the new single-shot mRNA COVID vaccines and is now scrambling to educate healthcare providers not to give children adult-strength jabs even while denying that overdosing is a safety risk.
Read MoreResearchers Question One-Size-Fits-All COVID Booster Strategy as FDA Circumvents Advisors
Federal health officials face a growing hurdle in their quest to persuade Americans of all ages and risk profiles to get updated COVID-19 boosters: strong proponents of vaccination.
From New England to the Bay Area, researchers voiced concerns to mainstream science and health publications in recent days that the one-size-fits-all model may be backfiring.
Read MoreAppeals Court Says FDA Denunciations of Ivermectin Look Like ‘Command,’ not Advice
The Food and Drug Administration (FDA) is claiming in federal court that it never told doctors not to prescribe ivermectin to treat COVID-19. Federal judges aren’t buying it, and state medical boards that rely heavily on FDA guidance continue to investigate doctors for such prescriptions.
Echoing a federal district judge nine months ago, a three-judge panel of the 5th U.S. Circuit Court of Appeals pressed a Justice Department lawyer to reconcile the FDA’s repeated public denunciations of ivermectin as an off-label COVID treatment with its insistence that the agency is not liable for resulting investigations of doctors who prescribe or promote it.
Read MoreMinneapolis Democrat Mayor Orders Police to Scale Back Arrests for Psychedelic Plants
Minneapolis Democrat Mayor Jacob Frey has ordered the city police department to stop enforcing most laws against using hallucinogenic plants.
Frey in announcing the order Friday pointed to the potential benefits of taking hallucinogenic plants to treat mental illnesses such as depression and post-traumatic stress disorder.
Read MoreWorld Health Organization Labels Aspartame as a Possible Cancer Cause, FDA Disagrees
A World Health Organization (WHO) committee has released a report that finds the well known sweetener aspartame is a possible cause of cancer.
The new classification is based on a review of “limited evidence.” The U.S. Food and Drug Administration (FDA), however, disagrees with the report released Thursday, according to NPR.
Read MoreLawmakers: FDA Delaying Investigation, Accountability over Baby Formula Shortage
U.S. House oversight lawmakers reviewing the FDA’s role in the baby formula shortage say the federal agency is dodging oversight and delaying providing answers.
Subcommittee on Health Care and Financial Services Chairwoman Lisa McClain, R-Mich., sent a letter to the U.S. Food and Drug Administration this week asking for interviews with FDA officials to get to the bottom of the baby formula crisis that rocked the U.S. last year.
Read MoreFDA Blasted for ‘Misleading’ mRNA COVID Vaccine Labels as ‘Sudden Death’ Research Mounts
Researchers around the world continue documenting potentially severe side effects from COVID-19 mRNA vaccines in certain demographics, but the Food and Drug Administration refuses to label them or even tell recipients the shots can’t stop transmission of an increasingly immune-evasive virus.
Autopsies and reviews of medical records revealed a much higher incidence of Pfizer and Moderna vaccine-associated heart deaths than officially categorized in South Korean, Japanese and Qatari government registries, particularly in younger people at lower risk from COVID. That echoes a German autopsy study of healthy people who died within 20 days of jabs.
Read MoreFDA Kicks Off Crackdown on ‘Dangerous’ Flavored Vapes Imported from China
Flavored, nicotine-packed vape products manufactured in China are becoming increasingly common among teenagers and raising health concerns.
The problem took off in February of 2020 after the U.S. Food and Drug Administration implemented a ban on the sale of many flavored vaping products, pushing compliant manufacturers out of the flavored market while some Chinese-based manufacturers continued to distribute and sell the now banned-products to American youth.
Read MoreCommentary: The FDA Must Partner with State AGs to Crack Down on Illegal Vapes and Keep Kids Safe
Millions of kids and teens in America are falling victim to an insidious campaign to get them hooked on illegal, disposable vapes that are made in China and intentionally marketed in youth-enticing flavors.
Read MoreCommentary: After Decades of Outsourcing to China, the U.S. is Running Out of Children’s Antibiotics
Acute shortages of orally delivered amoxicillin, penicillin and other children’s antibiotics throughout the 2022 and 2023 cold and flu season have made it difficult for doctors to treat normal childhood illnesses like ear infections, bronchitis, strep throat and rarer cases of infections caused after suffering Respiratory Syncytial Virus (RSV), and also sickle cell disease—for months.
The Food and Drug Administration (FDA) issued a warning about the amoxicillin shortage in Oct. 2022 just at the start of the cold and flu season. But since then, no statement has been issued by President Joe Biden about what appears to be an underreported public health crisis.
Read MoreFederal Judge Suspends FDA Approval of Abortion Pill
A federal judge on Friday suspended the Food and Drug Administration’s approval of mifepristone, effectively stopping sale of the drug nationwide.
Mifepristone is one of two drugs necessary for a sort of abortion cocktail that allows recipients to terminate a pregnancy via pill. The second drug, misoprostol, is available through a traditional prescription. The FDA in January announced a regulatory change to permit major pharmacy chains to carry the drug in stores as opposed to mail-order pharmacies or select clinics.
Read MoreFDA Sued for Withholding Information About Children’s Use of Hormone Treatments
On Monday, the United States Food and Drug Administration (FDA) was sued for allegedly withholding records detailing the off-label use of hormone treatments, such as puberty blockers, by underaged children.
Fox News reports that the lawsuit was filed by Stephen Miller’s America First Legal (AFL) group, which had previously made a Freedom of Information Act (FOIA) request back in September regarding the use of such hormone drugs on children under the Biden Administration. After the FDA refused to respond to the request, AFL filed their lawsuit.
Read MoreFDA Approves New Drug for Early Treatment of Alzheimer’s
The U.S. Food and Drug Administration on Friday approved a new drug to treat Alzheimer’s disease, with testing reportedly showing considerable success in helping patients with the debilitating condition.
The FDA said in a press release that it had approved the drug Leqembi for Alzheimer’s patients. The drug is “the second of a new category of medications approved for Alzheimer’s disease that target the fundamental pathophysiology of the disease,” the agency said.
Read MoreFDA Social Media Posts on COVID Under Legal, Medical Scrutiny for Misleading Claims
The FDA’s Twitter habits are getting scrutiny in court and from medical professionals as the feds seesaw between walking back their once-confident COVID-19 assertions and making sweeping new claims without providing evidence.
Having long ago conceded that COVID vaccines can’t stop viral transmission and that assertions to the contrary by President Biden among others were based on “hope” rather than science, the feds are now downplaying the influence of their social media to escape liability for allegedly violating statutory limits by interfering in medical judgments.
Read MoreGrowing Body of Evidence Disputes Claims That Puberty Blockers Are Safe, Reversible
Puberty blockers are widely touted by doctors and transgender activists as a safe and fully reversible way to pause puberty for children with gender identity issues, but a growing body of evidence is challenging those claims, according to The New York Times.
The drug prevents the surge in bone density that would normally occur during puberty, and patients can see lifelong bone issues that are never resolved, according to the Monday NYT article. Medical professionals are also challenging claims that the drug is reversible, arguing instead that blocking puberty permanently cements a child’s transgender identity and puts them on a path to lifelong biomedical intervention.
Read MoreBiden FDA Approves COVID Booster Shot for Children 5-11 Years Old Without Testing
Pfizer Inc. and BioNTech announced Wednesday the Biden Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for their “bivalent” mRNA COVID-19 booster shot for children aged 5-11 years without having completed clinical trials.
“As families across the country take part in fall festivities and plan for the upcoming holiday season, we aim to provide school-aged children with additional protection against the Omicron BA.4/BA.5 subvariants, which continue to account for more than 80% of cases in the U.S.,” said Pfizer CEO Albert Bourla. “Anticipating this need, we manufactured millions of booster doses, which will be made available, pending CDC recommendation, to help families stay up to date with COVID-19 vaccinations.”
Read MoreSen. Rand Paul: ‘Dr. Fauci Continues to Lie, Cover-Up, and Deny the Science to Promote Himself’
Senator Rand Paul (R-KY) once again battled with White House chief medical advisor Dr. Anthony Fauci Wednesday about whether children with natural immunity from a prior COVID infection should be injected with the mRNA shots the government has referred to as “vaccines.”
“Dr. Fauci continues to lie, cover-up, and deny the science to promote himself,” tweeted Paul about his tense exchange with Fauci during a Senate committee hearing whose central focus was the federal response to monkeypox.
Read MoreFDA Approves Most Expensive Drug in History
The U.S. Food and Drug Administration (FDA) on Wednesday approved a gene therapy for a rare blood disease which is set to reach the market at a record $2.8 million for a single dose, according to a press release by the therapy’s creator, Bluebird Bio.
Beta-thalassemia is an inherited blood disorder that causes a patient’s blood to fail to circulate oxygen through the body, according to the FDA press release concerning the approval. Bluebird’s new therapy, Zynteglo, infuses patients with cells that have a working copy of the gene responsible for the disorder, allowing the patient to produce blood that functions properly, according to a Bluebird press release.
Read MoreCommentary: Reducing Patient Access to New Medications Is Progressives’ Latest Medicare Price Fixing Scheme
As negotiations on their tax and spending bill continue, Senate Democrats are working on a legislative proposal to have the government fix the prices of Medicare prescription medications. Though the details of the 190-page amendment differ in certain respects from earlier versions, the indisputable result would be the same: Reduced patient access to prescription drugs.
Like most giant regulatory schemes, the draft proposal is characteristically complex with numerous provisions, including detailed data collection, new mandates, tax penalties on drug manufacturers, free vaccines, and a cap on out-of-pocket costs. But the heart of the bill is the creation of a Drug Price Negotiation Program administered by the Secretary of the U.S. Department of Health and Human Services (HHS).
Read MoreAppeals Court Grants Temporary Stay in Juul Fight Against FDA Banning Its E-Cigarettes
A federal appeals court on Friday granted a request for a temporary stay to vape manufacturer Juul Labs Inc. in its fight against the U.S. Food and Drug Administration’s ban of its e-cigarettes from being sold in the U.S.
The FDA issued marketing denial orders (MDOs) Thursday and said JUUL’s current inventory being sold in the U.S. “must be removed, or risk enforcement action.”
Read MoreFDA Quickly Authorizes COVID Shots for Infants and Young Children
The Food and Drug Administration (FDA) quickly authorized the Moderna and Pfizer-BioNTech COVID vaccine shots for infants and young children Friday, paving the way for the Centers for Disease Control and Prevention (CDC) advisory committee to vote on authorization over the weekend to allow the youngest children to get the shots as early as next week.
Per the press announcement by the FDA, the Emergency Use Authorization (EUA) for the Moderna COVID vaccine for older children and adults has been “amended” to “include use of the vaccine in individuals 6 months through 17 years of age,” while the EUA for the Pfizer COVID shot will now include use of the vaccine for babies as young as “6 months through 4 years of age.”
Read MoreRepublican Lawmakers Demand Answers from FDA on COVID Vaccines for Babies and Toddlers
A group of Republican lawmakers in the U.S. Senate and House are demanding answers from the Food and Drug Administration (FDA) and its vaccine panel regarding the Emergency Use Authorization (EUA) application for COVID vaccines in babies and young children under five years of age.
Though young children without other significant medical issues bear the least risk of serious illness from catching the COVID infection, White House COVID-19 Response Coordinator Dr. Ashish Jha is already planning for the distribution of COVID vaccines for babies and toddlers under five years of age as early as June 21 if the FDA and CDC approve.
Read MoreFDA Claims Whistleblower Report on Baby Formula Shortage Got Lost in Mailroom
An official with the Food and Drug Administration (FDA) claims that the reason the agency didn’t find a shocking whistleblower’s report on the baby formula shortage because the report was lost in the mailroom for at least four months.
The New York Post reports that the 34-page document had been sent to the FDA back in October, claiming that an Abbott Nutrition plant in Sturgis, Michigan was experiencing a shortage in product primarily due to increasingly unsanitary conditions. The FDA’s Frank Yiannas, Deputy Commissioner for Food Policy and Response, admits he didn’t actually see the report until February.
Read MoreDr. Robert Malone: ‘Rotten to the Core’ FDA Knew COVID Vaccines Could Spur Viral Reactivation, but Said Nothing
The Food and Drug Administration (FDA) was aware early on that the COVID vaccines could spur viral reactivation of diseases like the varicella-zoster virus (shingles) in some people, but chose not to disclose it, according to renowned vaccinologist and physician Dr. Robert Malone.
“They knew about the viral reactivation,” Malone declared during a recent panel discussion hosted by Del Bigtree with fellow Global COVID Summit physicians Dr. Ryan Cole, and Dr. Richard Urso.
Read MoreAnatomy of the American Baby Formula Shortage
At the outset of his presidency, Joe Biden promised competence by a bigger, better government. A few days ago, one of his loyal allies exposed a gross incompetence by federal officials on Biden’s watch that defied that promise and inflamed a baby formula shortage now panicking parents nationwide.
Rep. Rose DeLauro, D-Conn., a reliable liberal ally, unveiled documents showing the Biden Food and Drug Administration was alerted by a whistleblower last fall about potential contamination issues at the Abbott Nutrition baby formula factory in Michigan and failed for months to act aggressively.
Read MoreMinnesota Among the Hardest Hit as Bird Flu Outbreak Spreads to 25 States
The number of commercial and backyard flocks with confirmed avian flu increased by 36% in the past week, according to data on the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) website.
Three of the 57 new cases reported were in Missouri, bringing the state total to nine cases of Highly Pathogenic Avian Flu Influenza (HPAI) in seven counties—Bates, Dade, Gentry, Jasper, Lawrence, Ralls and Stoddard. Approximately 421,000 birds were in those flocks.
Read MoreCommentary: Biden Needs to Decide If COVID Is Still a ‘National Emergency’
The omicron variant may be nearing its peak in some states, but across the country it’s produced a dizzying array of conflicting signals on whether the nation should remain under a COVID national emergency or move on to an endemic “new normal.”
Comedian Bill Maher’s “I don’t want to live in your mask-paranoid world anymore” monologue went viral last week, just days after the Atlantic, the standard-bearer journal for the liberal intelligentsia, ran a story headlined: “COVID Parenting Has Passed the Point of Absurdity.” Accompanying the article was a black-and-white photo of a woman frozen in a more desperate and primal state of panic than the subject of Edvard Munch’s “The Scream.”
Omicron, for most people without co-morbidities, produces much milder symptoms than do the coronavirus’s previous variants, but it’s far more infectious, racing through schools, shutting down classrooms and forcing parents to consult their district’s ever-shifting COVID “decision trees” on a seemingly daily basis.
Read MoreFDA Pulls Authorization for Antibody Treatment, Refuses to Answer Questions
The U.S. Food and Drug Administration (FDA) Monday unexpectedly pulled its Emergency Use Authorization (EUA) for monoclonal antibody treatments for COVID-19, dealing a blow to states like Florida which have been using the treatment effectively for months.
“Without a shred of clinical data to support this action, Biden has forced trained medical professionals to choose between treating their patients or breaking the law,” Gov. Ron DeSantis (R) said in response to the FDA’s decree. “This indefensible edict takes treatment out of the hands of medical professionals and will cost some Americans their lives. There are real-world implications to Biden’s medical authoritarianism – Americans’ access to treatments is now subject to the whims of a failing president.”
Read MoreFDA Won’t Say Why Some Non-Approved Pfizer Vaccines Were Deemed ‘BLA-Compliant’
Through Ohio attorneys representing Miami University in a lawsuit against the school over its mandatory COVID-19 vaccine policy, The Ohio Star confirmed that at least none batches of Pfizer’s Emergency Use Authorization (EUA) vaccine were deemed Biologics License Application (BLA) compliant.
BLA compliance is typically reserved for Food and Drug Administration (FDA) approved drugs. That is the licensing procedure for drugs seeking to become FDA approved.
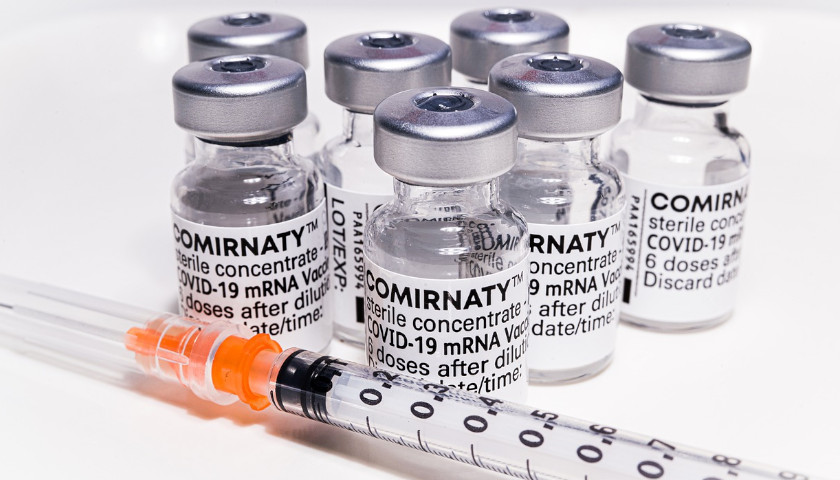
Read MorePfizer’s Comirnaty Available Abroad, Not in U.S.
New York-based Pfizer has sold and shipped hundreds of millions of doses of its Food and Drug Administration (FDA) approved COVID-19 vaccine Comirnaty to the European Union (EU) despite saying last week that it is not being shipped in the United States.
“Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced they will supply an additional 100 million doses of COMIRNATY®, the companies’ COVID-19 vaccine, to the 27 European Union (EU) member states in 2021,” Pfizer said in an April press release. “This announcement is a result of the European Commission’s (EC) decision to exercise its option to purchase an additional 100 million doses under its expanded Advanced Purchase Agreement signed on February 17, 2021. This brings the total number of doses to be delivered to the EU to 600 million.”
Read MoreFDA Authorized First COVID-19 Antiviral Pill in U.S.
The FDA on Wednesday authorized the first COVID-19 antiviral pill in the U.S.
The Pfizer pill, Paxlovid, will be prescribed for use in adults and children 12 and older who have mild to moderate virus symptom and at risk for severe disease or hospitalization, according to a Food and Drug Administration statement obtained by NBC News.
Read More6th Circuit Ruling Restoring Employer Vaccine Mandate Falsely Claims ‘Options Available to Combat COVID-19 Changed Significantly’ When ‘FDA Granted Approval to One Vaccine on August 23, 2021’
The majority opinion released on Friday by the 6th Circuit Court of Appeals, which restored the Biden administration’s Occupational Safety and Health Administration (OSHA) Emergency Temporary Standard (ETS) requiring employers with more than 100 employees to mandate that all employees take a COVID-19 vaccinefalsely asserts that Pfizer’s Food and Drug Administration (FDA) fully approved vaccine is currently available and in use among the general public.”
“At the same time, the options available to combat COVID-19 changed significantly: the FDA granted approval to one vaccine on August 23, 2021, and testing became more readily available,” the majority opinion asserts on page 24 of the ruling.
The majority opinion was written by Obama-appointed Judge Jane Branstretter Stranch of the United States Court of Appeals for the Sixth Circuit.
Read MoreFDA Claims It Needs 55 Years Before Revealing Data on Approval of Pfizer Vaccine
On Monday, the Food and Drug Administration (FDA) requested that the courts allow the agency to wait until the year 2076 to release all of the relevant documents regarding the approval of the vaccine developed by Pfizer and BioNTech, as reported by the Daily Caller.
The FDA made its request after a lawsuit was filed against the agency by the group Public Health Medical Professionals for Transparency (PHMPT). The PHMPT had previously made a Freedom of Information Act (FOIA) request on September 9th asking for the release of the vaccine approval documents; after the FDA denied the request, the group filed its lawsuit on September 16th.
The FDA concluded that there were roughly 329,000 pages in total that would qualify under this FOIA request. In its appeal to the courts, the agency said that, at most, employees would be able to “process and produce the non-exempt portions of responsive records at a rate of 500 pages per month.” Under this process, the FDA said that it would hand over prioritized documents to the plaintiff, and release non-exempt documents on a “rolling basis.”
Read MoreFDA Approves Moderna and Pfizer Boosters for Adults
The Food and Drug Administration (FDA) approved Moderna and Pfizer’s COVID-19 vaccines for booster shot use for adults in the U.S., the agency announced Friday,
The announcement was made just two months after the FDA first rejected the White House’s plan to administer booster shots to all adults the week of Sept. 20. FDA Acting Commissioner Janet Woodcock approved the booster without holding the usual public meeting to review the data, and the Centers for Disease Control and Prevention (CDC) will meet Friday afternoon to discuss the authorization, according to the FDA press release.
“Throughout the course of the COVID-19 pandemic, the FDA has worked to make timely public health decisions as the pandemic evolves. COVID-19 vaccines have proven to be the best and highly effective defense against COVID-19,” Woodcock said in the press release.
Read MoreBiden Announces Former Obama FDA Commissioner Robert Califf to Lead FDA Again
President Joe Biden on Friday announced the nomination of Robert Califf to head up the Food and Drug Administration again, urging the Senate to quickly confirm him a second time.
Califf, who served in the same role near the end of then-President Barack Obama’s second term, is “one of the most experienced clinical trialists in the country, and has the experience and expertise to lead the Food and Drug Administration during a critical time in our nation’s fight to put an end to the coronavirus pandemic,” Biden said in a statement announcing the pick.
“I am confident Dr. Califf will ensure that the FDA continues its science and data driven decision-making,” the president added, pointing out that Califf enjoyed “strong bipartisan support in the Senate in 2016.”
Read MoreMinnesota Teachers Union Wants State Holiday to Vaccinate Kids
Education Minnesota, the state’s far-left teachers union, wants lawmakers in St. Paul to “consider a state holiday for vaccinating students.”
Denise Specht, president of the union, thinks all eligible children should be vaccinated unless they have a “rare and legitimate medical reason” for not getting the shot.
“State and local leaders must be bold in their efforts to make this vaccine available to every student, no matter where they live or how much money they have,” she said.
Read More